Using Novel Biomarkers To Identify the Risk of Cardiovascular Disease
Biomarkers can provide valuable insights that can help us to pursue a long and healthy life.
Complete the form below to unlock access to ALL audio articles.
The concept of "building wellness" focuses on promoting health rather than just treating disease. Blood biomarkers are a powerful tool that we can use to gain a better understanding of individual health, helping us make more informed decisions and take targeted action to promote wellbeing and to reduce the risk of cardiovascular disease (CVD).
Early risk prediction is crucial to prevent cardiovascular events
Although CVD mortality has declined in recent decades, it remains the leading cause of death in Europe, accounting for 45% of total deaths.1 Early identification of persons at high risk long before the first development of cardiovascular abnormalities is crucial since it is preferable to prevent CVD instead of treating it.
Current CVD risk prediction is based on risk assessment scores built from the combination of traditional risk factors related to age, sex, smoking, blood pressure and total cholesterol. However, up to 20% of patients with coronary artery disease (the most common type of CVD) have none of the traditional risk factors.2 Novel biomarkers can add value to existing models to improve the accuracy of risk estimation for CVD.3
Biomarkers in cardiovascular risk assessment
To be effective in cardiovascular risk assessment; a biomarker must be able to accurately identify individuals at risk. Furthermore, this identification must be reproducible across a population, and, because of this identification, early intervention should have a therapeutic impact.3
Multiple large population-based studies have identified innovative diagnostic biomarkers that were able to specifically identify individuals that later developed or died from CVD.
The role of the biomarkers as predictors for CVD risk is based on their crucial functions in:
- Antioxidant defense (Selenoprotein-P [Sel-P])4-8
- Lipid metabolism (Proneurotensin [pro-NT])9
- Cell growth and regeneration processes (human growth hormone [hGH])10
These biomarkers are critical for the development of CVD, when dysregulated.
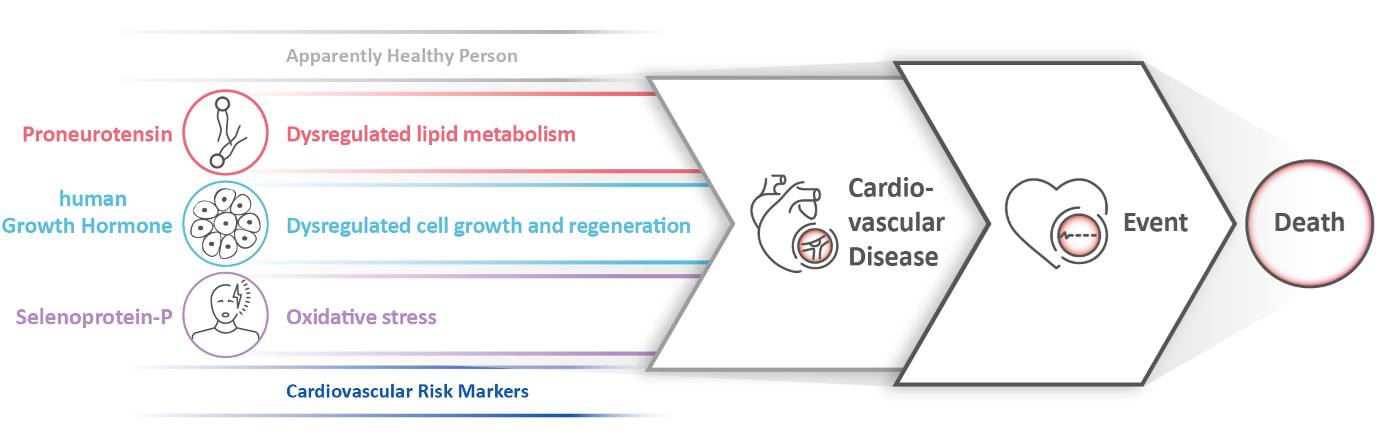
Figure 1: The roles of biomarkers in the development of cardiovascular diseases. © SphingoTec
Using neurotensin to understand the link between fast food and heart disease
Worldwide, over half of adults frequently eat unhealthy fast food.11 Most clinicians recognize that fast food consumption is associated with premature heart disease; the consensus is that the saturated fats in these foods are increasing CVD risk.
Neurotensin is a biomarker for the risk prediction of CVD as a result of its decisive role in the regulation of lipid absorption. The quantity of neurotensin in the blood indicates the body’s capacity to absorb and utilize saturated fatty acids. However, due to the instability of neurotensin in vivo and in vitro, proneurotensin (pro-NT), a stable fragment of the precursor molecule, is used as a surrogate marker. Studies have shown that women with a high pro-NT level when fasting are up to 65% more at risk of a CVD event compared to women with a low level, and their risk of dying from CVD increases over 200%, regardless of body weight.12-16
By warning individuals with high pro-NT levels that they are at increased risk of CVD due to unhealthy nutritional behaviors, it can motivate them to make lifestyle adjustments. For example, consuming fewer processed and more nutrient-rich foods may be a good start.17 In particular, a Mediterranean diet low in saturated fats and foods with a low glycemic index could have a positive impact on cardiovascular health.18 The effects of these lifestyle changes can be monitored with biomarkers to encourage long-lasting nutritional habits to reduce the risk of CVD.
Human growth hormone and its power to reduce CVD risk
Human growth hormone (hGH) stimulates growth, cell production and cell regeneration. Routinely, hGH is used in the diagnosis of growth disorders. However, elevated hGH concentrations are also associated with an increased risk of CVD, opening new possibilities for the early identification of at-risk men.19-24
hGH can be reliably measured in fasting subjects aged 40 years and older. Several studies have shown that higher levels of hGH are significantly associated with increased risk of cardiovascular morbidity and mortality.25-27 Men with a highly elevated hGH level have up to three times the risk of CVD mortality compared to men with a low hGH level. Therefore, monitoring hGH levels can help improve risk prediction beyond traditional cardiovascular risk factors.
In addition to its role in primary CVD prevention, hGH also improves secondary prevention as a biomarker in the prediction of the efficacy of antihypertensive drugs. These drugs, such as ACEi/ARB or beta-blockers, are given to patients after myocardial infarction to prevent the recurrence of major adverse cardiac events (MACE).26
The importance of the trace element selenium
Selenium is an essential trace element important for our health; it is found in the body in the form of selenoproteins which have roles in antioxidant defense, immune system support and the cardiovascular system, among others.
Undersupply of selenium increases the oxidative stress within the cardiovascular system and is still an unrecognized health problem.28 Large parts of Europe, Asia, Australia and Africa have selenium-poor soils, and consequently, selenium-poor food.27 Studies have shown that up to 20% of the Northern European population suffers from selenium deficiency.29 Dietary intake of selenium is not always sufficient for complete selenoprotein biosynthesis, and while selenium deficiency is a well-known cause of thyroid dysfunction and tumors, the importance of selenium for cardiovascular health is underestimated.
Selenoprotein-P (Sel-P) is the most abundant selenoprotein in the blood. It plays a crucial role in the transport and delivery of selenium to peripheral tissues for the synthesis of other selenoproteins, making it an ideal indicator of bioavailable selenium.4 Studies suggest that individuals with the lowest baseline Sel-P levels have about 40% increased risk of a cardiovascular event and a 60% higher mortality risk than subjects with normal Sel-P levels.27,29
Identification of Sel-P deficiency allows targeted prevention strategies by selenium supplementation and, thus, a reduction of CVD. Sel-P deficiency is still an unrecognized independent risk factor in apparently healthy elderly women and men for a first cardiovascular event and cardiovascular mortality. But by knowing its level, we can avoid CVD by simple selenium supplementation with regular monitoring.
The pursuit of a long and healthy life
Whether we are looking to prevent disease or manage existing health conditions, biomarkers can provide valuable insights that can help us to pursue a long and healthy life.
Biomarkers play a valuable role in providing insights into one's health status and guiding decision-making processes. They forerun CVD prevention strategies and can push individuals to attain optimal wellbeing. Therefore, it is essential to run a regular biomarker check and to incorporate lifestyle adjustments and modify diets accordingly. This will break us away from entrenched habits to achieve a comprehensive and sustainable state of health.
References:
1. European Cardiovascular Disease Statistics 2017. European Heart Network. https://ehnheart.org/cvd-statistics/cvd-statistics-2017.html#:~:text=European%20Cardiovascular%20Disease%20Statistics%202017,all%20deaths%20in%20the%20EU. Published February 2017. Accessed October 10, 2023.
2. Hozawa A, Folsom AR, Sharrett AR, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects--Atherosclerosis Risk in Communities Study. Arch Intern Med. 2007;167(6):573–579. doi: 10.1001/archinte.167.6.573.
3. Wang J, Tan GJ, Han LN, Bai YY, He M, Liu HB. Novel biomarkers for cardiovascular risk prediction. J Geriatr Cardiol. 2017;14(2):135–150. doi: 10.11909/j.issn.1671-5411.2017.02.008.
4. Burk RF, Hill KE. Regulation of selenium metabolism and transport. Annu Rev Nutr. 2015;35:109–134. doi: 10.1146/annurev-nutr-071714-034250.
5. Saito Y, Hayashi T, Tanaka A, Watanabe Y, Suzuki M, Saito E, et al. Selenoprotein P in human plasma as an extracellular phospholipid hydroperoxide glutathione peroxidase. Isolation and enzymatic characterization of human selenoprotein P. J Biol Chem. 1999;274(5):2866–2871. doi: 10.1074/jbc.274.5.2866.
6. Traulsen H, Steinbrenner H, Buchczyk DP, Klotz LO, Sies H. Selenoprotein P protects low-density lipoprotein against oxidation. Free Radic Res. 2004;38(2):123–128. doi: 10.1080/10715760320001634852.
7. Arteel GE, Mostert V, Oubrahim H, Briviba K, Abel J, Sies H. Protection by selenoprotein P in human plasma against peroxynitrite-mediated oxidation and nitration. Biol Chem. 1998;379(8–9):1201–1205. https://europepmc.org/article/med/9792455. Accessed October 10, 2023.
8. Sasakura C, Suzuki KT. Biological interaction between transition metals (Ag, Cd and Hg), selenide/sulfide and selenoprotein P. J Inorg Biochem. 1998;71(3-4):159–62. doi: 10.1016/s0162-0134(98)10048-x.
9. Barchetta I, Baroni MG, Melander O, Cavallo MG. New Insights in the control of fat homeostasis: The role of neurotensin. Int J Mol Sci. 2022;23(4). doi: 10.3390/ijms23042209.
10. Guevara-Aguirre J, Guevara A, Palacios I, Perez M, Procel P, Teran E. GH and GHR signaling in human disease. Growth Horm IGF Res. 2018;38:34–38. doi: 10.1016/j.ghir.2017.12.006.
11. Braithwaite I, Stewart AW, Hancox RJ, Beasley R, Murphy R, Mitchell EA, et al. Fast-food consumption and body mass index in children and adolescents: an international cross-sectional study. BMJ Open. 2014;4(12):e005813. doi: 10.1136/bmjopen-2014-005813.
12. Melander O, Maisel AS, Almgren P, Manjer J, Belting M, Hedblad B, et al. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA. 2012;308(14):1469–75. doi: 10.1001/jama.2012.12998.
13. Januzzi JL, Jr., Lyass A, Liu Y, Gaggin H, Trebnick A, Maisel AS, et al. Circulating proneurotensin concentrations and cardiovascular disease events in the community: The Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2016;36(8):1692–1697. doi: 10.1161/ATVBAHA.116.307847.
14. Wettersten N, Cushman M, Howard VJ, Hartmann O, Filippatos G, Beri N, et al. Usefulness of proneurotensin to predict cardiovascular and all-cause mortality in a United States population (from the Reasons for Geographic and Racial Differences in Stroke Study). Am J Cardiol. 2018;122(1):26–32. doi: 10.1016/j.amjcard.2018.03.009.
15. Fawad A, Bergmann A, Struck J, Nilsson PM, Orho-Melander M, Melander O. Proneurotensin predicts cardiovascular disease in an elderly population. J Clin Endocrinol Metab. 2018;103(5):1940–1947. doi: 10.1210/jc.2017-02424.
16. Nicoli CD, Wettersten N, Judd SE, Howard G, Howard VJ, Struck J, et al. Pro-neurotensin/neuromedin N and risk of ischemic stroke: The REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Vasc Med. 2020;25(6):534–540. doi: 10.1177/1358863X20957406.
17. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678.
18. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34. doi: 10.1056/NEJMoa1800389.
19. Orme SM, McNally RJ, Cartwright RA, Belchetz PE. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab. 1998;83(8):2730–2734. doi: 10.1210/jcem.83.8.5007.
20. Melmed S, Casanueva FF, Klibanski A, Bronstein MD, Chanson P, Lamberts SW, et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary. 2013;16(3):294-302. doi: 10.1007/s11102-012-0420-x.
21. Colao A, Marzullo P, Di Somma C, Lombardi G. Growth hormone and the heart. Clin Endocrinol (Oxf). 2001;54(2):137–54. doi: 10.1046/j.1365-2265.2001.01218.x.
22. Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341(11):785–792. doi: 10.1056/NEJM199909093411102.
23. Maison P, Balkau B, Simon D, Chanson P, Rosselin G, Eschwege E. Growth hormone as a risk for premature mortality in healthy subjects: data from the Paris prospective study. BMJ. 1998;316(7138):1132–1133. doi: 10.1136/bmj.316.7138.1132.
24. Hallengren E, Almgren P, Engstrom G, Hedblad B, Persson M, Suhr J, et al. Fasting levels of high-sensitivity growth hormone predict cardiovascular morbidity and mortality: the Malmö diet and cancer study. J Am Coll Cardiol. 2014;64(14):1452–1460. doi: 10.1016/j.jacc.2014.03.063.
25. Ng LL, Bhandari SS, Sandhu JK, Quinn PA, Squire IB, Davies JE, et al. Growth hormone for risk stratification and effects of therapy in acute myocardial infarction. Biomarkers. 2015;20(6-7):371–375. doi: 10.3109/1354750X.2015.1093031.
26. Wettersten N, Mital R, Cushman M, Howard G, Judd SE, Howard VJ, et al. Growth hormone concentration and risk of all-cause and cardiovascular mortality: The REasons for Geographic And Racial Disparities in Stroke (REGARDS) study. Atherosclerosis. 2022;359:20–26. doi: 10.1016/j.atherosclerosis.2022.09.004.
27. Combs GF, Jr. Selenium in global food systems. Br J Nutr. 2001;85(5):517–547. doi: 10.1079/bjn2000280.
28. Shimada BK, Alfulaij N, Seale LA. The impact of selenium deficiency on cardiovascular function. Int J Mol Sci. 2021;22(19):10713. doi: 10.3390/ijms221910713.
29. Schomburg L, Orho-Melander M, Struck J, Bergmann A, Melander O. Selenoprotein-P deficiency predicts cardiovascular disease and death. Nutrients. 2019;11(8). doi: 10.3390/nu11081852


