Deciphering SARS-CoV-2 Transmission With Serology Surveillance

Complete the form below to unlock access to ALL audio articles.
Some of the information in this article is based on research findings that are yet to be peer-reviewed. Results are therefore regarded as preliminary and should be interpreted as such. Find out about the role of the peer review process in research here. For further information, please contact the cited source.
In many countries, diagnostic testing to detect active SARS-CoV-2 infections has played an important role in the management of coronavirus disease-19 (COVID-19). Mostly using real-time reverse transcription PCR (rRT-PCR), tests for active infection have informed measures related to contact tracing, self-isolation, and healthcare management on an individual level.1
Using rRT-PCR tests alone, however, it is difficult to derive accurate population-level insights on true disease burden. Sampling biases, variable testing capacity and multiple other factors can easily limit data interpretation. Also, many COVID-19 infections are mild or subclinical, there are many positive cases which go unnoticed or unreported.2 To determine true infection rates, therefore, supplementary testing approaches are needed. In this article, we explore how seroprevalence studies, also known as serosurveys, provide a means of understanding community transmission of SARS-CoV-2. We also discuss the challenges of interpreting serological results and identifying people who had mild infections.
Antibodies used in COVID-19 serosurveys
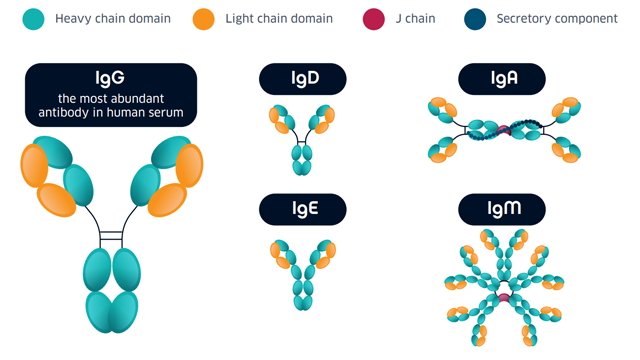
Figure 1: Antibody classes.
Not all of these isotypes are measured in serological assays; IgA has a central role in mucosal immunity4 and little is known about the significance of its dynamics in relation to COVID-19, while the function of IgD in general is poorly understood5, and there is no serological evidence for the induction of IgE production in people with COVID-19 (as of December 2020).4 Most serological assays are based on the measurement of IgG and/or IgM. As discussed below, while antibody tests are an important tool for understanding community transmission, it is important to consider their limitations when interpreting results. In general, IgG and IgM measurements are used to indicate:
- Early/current or recent infection: IgM is the first response antibody and becomes undetectable weeks to months following infection. A positive IgM test may be indicative of a more recent infection, although the dynamics of the IgM antibody response are not well defined.
- Previous infection: IgG antibodies usually develop within 7 to 10 days after COVID-19 symptom onset and remain in the blood after the infection has passed.
How data from serosurveys can be used
Across the globe, over 100 serosurveys have been conducted to estimate the true prevalence of infection. Authors claim surveillance can inform disease trends, resource allocation, and effectiveness of community interventions by providing an estimate of the extent of an outbreak and enabling outbreak dynamics to be characterized in greater detail.6 Theoretically, accurate mapping and tracking allow epidemiologists and public health specialists to take evidence-based action upon the identification of hotspots and at-risk groups, and also see how serosurvey data changes over time within the same group.7,8 Data from SARS-CoV-2 prevalence estimates can be stratified by sex, age group, and geographical region to inform3,8:
- Social distancing strategies to balance public health and economic interests
- Personal protective equipment (PPE) distribution based on predicted demand
- Changes in travel restrictions
- Strategic decisions on essential staffing in hospitals and other health care facilities
Immunologic Testing for COVID-19

Preventive measures, accurate disease diagnostics and effective contact tracing are still considered fundamental in efforts to combat the SARS-CoV-2 pandemic. Immunologic tests can be performed under common clinical settings and the results are available in a matter of hours, which significantly expedites diagnostic practices. Download this whitepaper to discover different testing methods and the impact of COVID-19 variants and antibody cross reactivity.
View WhitepaperSponsored Content
Challenges in interpreting serological data
In practice, interpreting and comparing data from serosurveys can be difficult, with studies varying in size, diagnostic test and sampling approach. Also, the lag between data collection and reporting is a major challenge, explains Jill Foster, professor in the Division of Pediatric Infectious Diseases and Immunology at the University of Minnesota Medical School.
“My feeling on data is that it is almost definitely good to have, but the question is, what should we do with it? Serosurveys will continue to be important for providing us a snapshot – but not a full picture – of what is occurring and will need to be interpreted carefully. Big swings upwards will alert us that something might be happening, i.e., that we need to dig deeper to find an outbreak or a micro-outbreak deserving of more intensive track and trace efforts. One needs to keep in mind, however, that by the time that an antibody change is detected, it may be too late to intervene. A more useful approach would be to complex it with data from molecular wastewater monitoring to provide more real-time information from two sources of data.”
As Peeling and Olliaro (2020) highlighted, serology tests must be highly sensitive and specific if they are to inform public health measures and controls, and should be thoroughly assessed to minimize misclassifying people.7 They also add that estimating the proportion of the population that has been infected is made even more difficult if testing strategies are changed over time. As less costly and invasive tests become available, strategies are indeed highly likely to change. The list of commercially available COVID-19 diagnostic tests continues to grow, as seen in this coronavirus test tracker which documents serological tests to have received regulatory approval across the US, Europe and Asia.
The challenge of detecting mild cases of COVID-19
There have been reports of patients who have contracted a mild form of COVID-19, yet have not seroconverted. In other words, they do not have detectable amounts of serum IgG.9 To investigate this apparent lack of seroconversion, Magnus Gisslén, professor in the Department of Infectious Diseases at the University of Gothenburg, led a study investigating serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection, measured using two commercially available serological assays. The report, now published in PLOS ONE, describes the concentration and time course of SARS-CoV-2-specific IgG antibodies in serum of patients with severe (n=15) and mild symptoms (n=32) of COVID-19.10
“The lack of seroconversion in the 10% of patients with mild disease was due to the sensitivity and specificity of the commercial assays,” explains Gisslén. “Using the more sensitive assays, we saw they had indeed developed antibodies, i.e., seroconverted. To avoid the risk of false positives, commercial tests need to be calibrated in such a way that risks false negatives.” By highlighting missed positive cases, the study has major implications for the interpretation of COVID-19 seroprevalence studies.
In the three patients with mild COVID-19 who did not develop detectable anti-SARS-CoV-2 IgG in serum, neutralizing antibodies (NAbs) were detectable. One might ask, why is NAb detection not part of standard commercial tests? Is this a feasible measurement to incorporate in future commercial tests? Not quite, says Gisslén: “The analysis of neutralizing antibodies is highly laborious and uses live virus, which requires the use of special labs. It is therefore not feasible to use NAbs as a clinical routine test. However, antibody tests could be developed to closely match the results that could be obtained from a virus neutralization test. For example, we could measure antibodies against the receptor-binding domain (RBD) of the spike protein. Such commercial tests are becoming increasingly available.”
Assessing the risk of reinfection: an open question
Early in the pandemic, it was thought that antibody tests might serve as the basis for an “immunity passport” that would enable individuals to continue with their daily lives, assuming they are protected against reinfection. Without data from thorough, long-term reinfection studies, however, such an assumption cannot be made safely. As a result, this continues to be an area of active research.
Interim preprint results from a large-scale, multi-center prospective study of healthcare workers in the UK provide an insight; of 6614 participants who were antibody-positive or had a history of testing PCR/antibody-positive to SARS-CoV-2, 44 reinfections (two probable, 42 possible) were detected within a five-month period. The incidence density per 100,000 person days was 3.3 in the positive cohort, compared with 22.4 in the negative cohort, leading the authors to conclude that prior SARS-CoV-2 infection appears to protect most individuals against reinfection for at least five months. Ongoing studies aim to address how long antibody protection lasts after infection – and will now need to take new virus variants into consideration.
Serosurveys are valuable, but handle with care
As summarized by an editorial board member in the journal Public Health in Practice, serological tests are not perfect, but they provide much-needed estimates of the fraction of the population with SARS-CoV-2.3 Their value is heavily influenced by their diagnostic accuracy and the sampling strategy employed, and therefore it is important to take study limitations into consideration when interpreting results. Overall, the path to understanding COVID-19 dynamics might not be straightforward, but it is forcing significant progress in diagnostic developments. Hopefully, this progress will spill over, and benefit the diagnosis and management of other diseases in the future.
References
1. Mallett S, Allen AJ, Graziadio S, et al. At what times during infection is SARS-CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data. BMC Med. 2020;18(1):346. doi:10.1186/s12916-020-01810-8
2. Wu SL, Mertens AN, Crider YS, et al. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat Commun. 2020;11(1):4507. doi:10.1038/s41467-020-18272-4
3. Kritsotakis EI. On the importance of population-based serological surveys of SARS-CoV-2 without overlooking their inherent uncertainties. Public Health in Practice. 2020;1:100013. doi:10.1016/j.puhip.2020.100013
4. Galipeau Y, Greig M, Liu G, Driedger M, Langlois M-A. Humoral Responses and Serological Assays in SARS-CoV-2 Infections. Front Immunol. 2020;11:610688. doi:10.3389/fimmu.2020.610688
5. Chen K, Cerutti A. New insights into the enigma of immunoglobulin D: Regulation and function of IgD. Immunological Reviews. 2010;237(1):160-179. doi:10.1111/j.1600-065X.2010.00929.x
6. Anand S, Montez-Rath M, Han J, et al. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. The Lancet. 2020;396(10259):1335-1344. doi:10.1016/S0140-6736(20)32009-2
7. Peeling RW, Olliaro PL. The time to do serosurveys for COVID-19 is now. The Lancet Respiratory Medicine. 2020;8(9):836-838. doi:10.1016/S2213-2600(20)30313-1
8. Bajema KL, Wiegand RE, Cuffe K, et al. Estimated SARS-CoV-2 Seroprevalence in the US as of September 2020. JAMA Intern Med. Published online November 24, 2020. doi:10.1001/jamainternmed.2020.7976
9. Burgess S, Ponsford MJ, Gill D. Are we underestimating seroprevalence of SARS-CoV-2? BMJ. Published online September 3, 2020:m3364. doi:10.1136/bmj.m3364
10. Marklund E, Leach S, Axelsson H, et al. Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders. Walsh SR, ed. PLOS ONE. 2020;15(10):e0241104. doi:10.1371/journal.pone.0241104


