Care in the Community – How Biofilms Improve Bacterial Survival

Complete the form below to unlock access to ALL audio articles.
A single bacterial cell on its own is vulnerable to attack by immune mediators, antimicrobials such as antibiotics and even other bacteria, and like many living things they can benefit from safety in numbers. But bacteria take communal living to a whole new level, forming veritable civilizations - the bacterial biofilm.
What is a biofilm and how does a biofilm form?
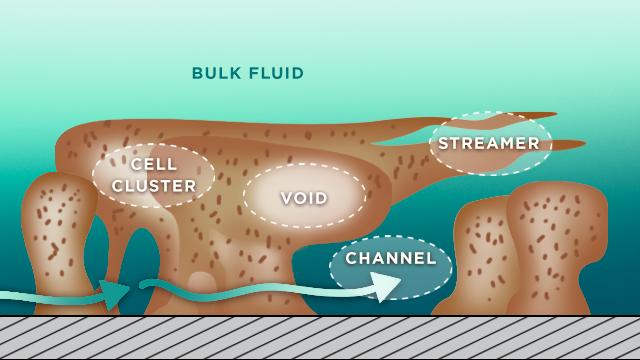
Biofilms in both Gram-negative and Gram-positive bacteria are highly organized, functionally heterogeneic communities of bacteria. Their self-made hydrated polymeric matrix has channels for nutrient acquisition and waste disposal. This structure not only provides protection from harm but allows them to exploit the available resources, so they can persist under stressful conditions where oxygen or nutrients for example may be in short supply.
Triggers for biofilm formation vary between bacterial species and are not well understood in many. However, broadly speaking it is thought to be a survival mode response to stressors in the surrounding environment.
A whole host of bacterial species, including Streptococcus, Neisseria and Actinomyces embrace a biofilm mode of growth when conditions are right. Some bacterial species are better adapted to different functions, such as surface attachment, than others and consequently it is not unusual for multiple species to be present in a single biofilm. The upside is that cooperation in these complex interspecies networks can often produce synergistic benefits to all involved.
Biofilms are found in diverse corners of the globe, from medical devices and food preparation surfaces to rock pools and even your mouth. The robust nature of the bacterial biofilm makes it hard to remove once established as antimicrobial agents struggle to penetrate and eradicate the bacteria within. Consequently, whilst they provide a haven for bacterial survival, they can also be very challenging to remove where they are unwanted. It has been estimated that 80% of human infections begin with polymicrobial biofilms so it is clear that they are a significant health issue.
Biofilms are challenging in surgical sites and medical devices
Whilst our skin protects us from invading microbes, surgical interventions break this barrier. The insertion of medical devices be they totally internal, such as joint replacements or pacemakers, or bridging the gap between the body and environment, such as catheters and ventilators, provides further opportunity for bacterial invaders to establish persistent infections.
Dr K. Scott Phillips, Commander at the Center for Devices and Radiological Health (CDRH), United States Food and Drug Administration commented “We have a lot more awareness of the skin microbiome and the role that skin, nasal, and oral biofilm communities can play in seeding infections. Whenever you break the skin barrier while implanting, inserting, or injecting a medical device, you risk contamination from the skin. Indwelling devices face similar risks but are even more challenging because you have a constant opening to the outside world.”
As well as providing a route of entry, medical devices, which are themselves foreign to our body, can make infection clearance more challenging for our immune systems. “One study in humans showed that it only took 100 Staphylococci bacterial cells to cause an infection when there is a foreign material, whereas it took over 10,000 times more cells of the same type to cause an infection without a foreign material” commented Dr Phillips.
The protected nature of a biofilm and slow growth of cells at its heart make treatment of biofilms with antimicrobials challenging. Genetic exchange between the bacteria within the biofilm means that antibiotic resistance genes may spread through the population. “Experiments in our laboratory have shown that bacteria in biofilms survive treatment with some antibiotics at concentrations that are 10,000 x that which is normally considered a minimal inhibitory concentration (MIC) for the planktonic bacterial cells” stressed Dr Phillips.
Injectable dermal fillers, a primarily cosmetic medical intervention, have been associated with terrible infections and consequent disfiguration when used improperly. As the needle passes through the skin, it can pick up bacteria which are then delivered into the filler, setting up infection in the tissue. “Our research showed that the elastic properties of hydrogel medical devices could really impact how bacteria stick to them, and possibly create a niche for biofilm infections. We were very surprised to find that small differences in how soft a hydrogel is could cause a 10,000-fold difference in the amount of bacteria that stick to it.”
To try and reduce the risks of this procedure, Dr Phillip’s team sought to better understand how contamination came about. “First we created a pigskin model to study different types of injections that surgeons use. We found that some injections are more likely to result in bacterial contamination than others. Then we studied the skin preparation used for these injections. We found that just wiping a few times with an alcohol wipe is not sufficient to eradicate harmful organisms in biofilms on the skin.”
To reduce infection, effective skin decontamination is vital for any surgical procedure or device implantation. “Using a combination of a sonication brush and a probiotic extract, we were able to reduce the amount of bacteria in biofilms on pig skin by about 1,000 times more than just using an alcohol wipe.” For people living with permanent implants, such as amputees who have osseointegrated implants for prostheses attachment, this could be life-changing
Speaking on the impact of their work Dr Phillips said “We want to encourage changes in clinical practice that will reduce the chance of infection and encourage development of products that are less likely to harbor biofilms. The information that we are learning from biofilm research is being fed into regulatory science and decisions through the robust CDRH scientific expert consult process, educational training, and standards development.”
“Biofilms pose unique challenges for regulatory science, so we have tried to use our research to answer many of those questions through improved in vitro model development, better biofilm quantification methods, and even tools for detecting biofilm in the clinic.”
Considering treatment approaches for the future Dr Phillips enthused “We are excited about the potential of what we call “combination” strategies, which include physical forces in combination with chemical or biological components, that may help reduce our reliance on precious antibiotics.”
Oral biofilms – a help and a hindrance
The mouth, whilst providing habitable niches and a potential ready source of nutrients, can be a challenging environment for bacterial survival. Shear forces from saliva, food and drink and associated sudden temperature changes are just some of the challenges facing any bacteria wishing to take up residence. Consequently, the protection offered by a biofilm mode of growth leads to an abundance in our mouths.
Many of the bacteria living there are harmless commensals and form part of a healthy mouth, excluding potentially harmful invaders and even contributing to normal tissue and immune system development. Dr Angela Nobbs, Senior Lecturer and Group Leader in Oral Microbiology within the University of Bristol Dental School commented “Problems arise, however, when this balance is disrupted, leading to a dysbiotic community that may then promote the onset of site-specific disease, such as dental caries or periodontitis.” There is no specific “guest list” for welcome and unwelcome colonizers in our mouths. Dr Nobbs went on to say “Microbial diversity in communities colonizing tooth enamel, mucosal surfaces, etc., is individual-specific and site-specific. Nonetheless, there is a conserved oral community in healthy mouths at the genus level.”
Triggers for transitioning from a healthy to unhealthy population can include ill health of the individual but can also be factors as simple as diet. Frequent intake of fermentable carbohydrate for example is the predominant trigger for dental caries.
To reduce harmful biofilm formation and improve treatments, it is vital that scientists have a good understanding of the pathogenic processes and interactions that are occurring in the mouth at the molecular level and can move beyond observational correlations.
One aspect that is of particular interest to the group is the role that extracellular DNA (eDNA) plays in facilitating and stabilizing oral plaque biofilms, with in vitro studies already having shown that DNases can successfully disrupt mature oral biofilms.
“It’s important that advanced models, both in vitro and in vivo, are developed in which polymicrobial communities and their interplay with host systems can be better studied and in a way that more closely mimics the human ecosystem.”
“Given its complexity, a coordinated and combined approach utilizing -omic technologies, mathematical modelling of community dynamics, advanced multispecies models, and molecular characterization of intermicrobial interactions will ultimately be needed to fully elucidate the critical drivers of oral plaque biofilm community composition and pathogenic potential.” Dr Nobbs stressed.
Considering the bigger picture Dr Nobbs concluded “The recalcitrance of biofilms to antimicrobial agents and their role as ‘hotspots’ for genetic exchange also means that the development of novel strategies to disrupt biofilm formation will likely make a significant contribution to ongoing efforts to combat the growing crisis of antimicrobial resistance”.


